Dynamics of chaperone interactions
with client proteins during folding and secretion
|
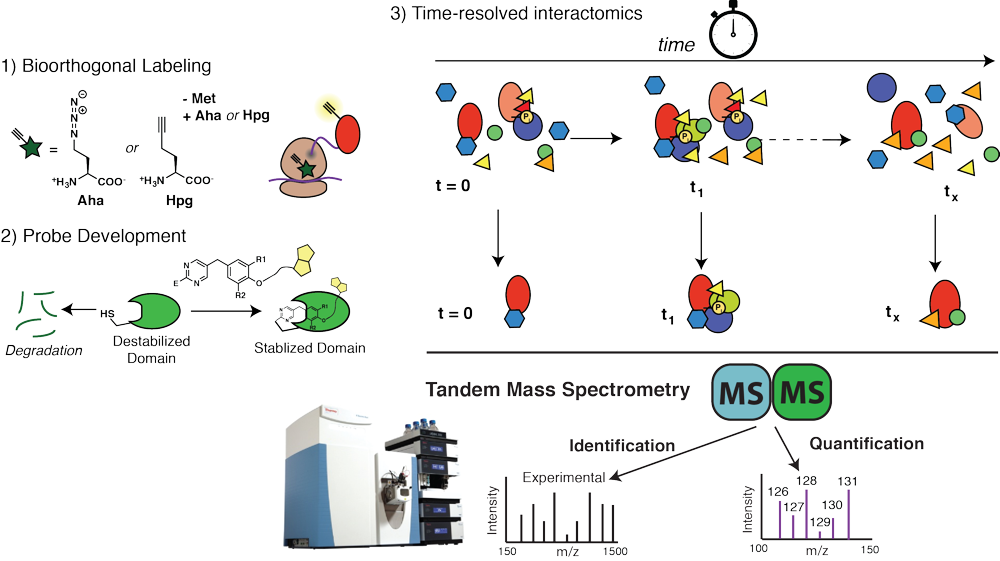
Protein folding and assembly inside cells rely on the coordinated action of chaperones, co- chaperones and other quality control factors that comprise the proteostasis network. Our goal is to monitor the sequential engagement and interaction dynamics between client proteins and proteostasis network components during cellular protein folding. We develop mass spectrometry tools for time-resolved interactomics coupled to chemical biology labeling approaches. Using these approaches, we explore how altered progression of client proteins through proteostasis pathways can affect the quality of the folded proteins and lead to protein misfolding diseases. This will reveal previously invisible mechanisms responsible for the quality control defects in protein misfolding diseases and identify new strategies for disease intervention. |